1-800-GET-THIN LLC has been warned, along with eight surgical centers in California, to stop misleading people in their advertising about the Lap-Band, a medical device approved by the FDA to help obese adults lose weight. The Food and Drug Administration (FDA) accused the advertisers of omitting important negative data on the Lap-Band, such as contraindications, warnings, precautions, and potential side-effects.
The following have been sent warning letters by the FDA:
- 1-800-GET-THIN
- Bakersfield Surgery Institute Inc
- Beverly Hills Surgery Center
- Cosmopolitan Plastic & Reconstructive Surgery
- Palmdale Ambulatory Center
- San Diego Ambulatory Center LLC
- Top Surgeons LLC
- Valencia Ambulatory Center LLC
- Valley Surgical Center
The thrust of the message is that the Lap-Band, being a restricted medical device, is being misbranded due to misleading advertising.
The FDA also mentioned that any negative information regarding the device that appears on the advert is in too-small-a-font size.
Steve Silverman, director of the Office of Compliance in the FDA’s Center for Devices and Radiological Health, said:
“The FDA takes seriously its responsibility to protect consumers from products promoted without adequate warnings. It’s particularly troublesome when advertisements don’t communicate the serious risks associated with medical devices.”
The companies have been told by the FDA that they could face fines or product seizure if they do not address the concerns included in the Warning Letters.
The Lap-Band, also referred to as a (Laparoscopic) Adjustable Gastric Band, is an inflatable silicone medical device that is surgically placed around the top portion of the stomach – it is used to treat patients with a BMI (body mass index) of 30 to 40 (obese).
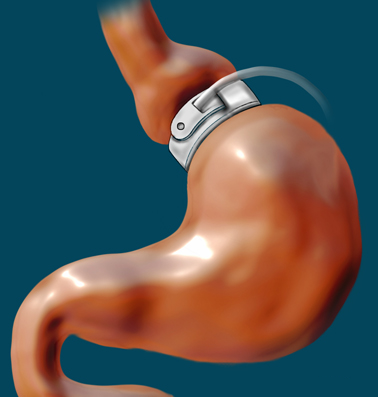
The Lap-Band, also known as the Adjustable Gastric Band
The obese patient candidate for the Lap-Band often also has an obesity related medical condition, such as high blood pressure (hypertension) or diabetes type 2. The doctor may consider gastric banding when other therapies were not effective, such as dieting, exercise or behavior change.
A patient who is willing to undergo a Lap-Band procedure has to be committed to making significant lifestyle and eating habit changes.
Kimber Richter, M.D., deputy director for medical affairs in the Office of Compliance in the FDA’s Center for Devices and Radiological Health, said:
“The decision to undergo a gastric banding procedure should be done in close consultation between a patient and his or her health care provider. It is important for the patient to fully understand both the risks and the benefits of the procedure and for the health care provider to be sure the procedure is appropriate for the patient.”
Patients under the age of 18 years are not allowed to have the Lap-Band placed. The FDA says it is crucial that patients and doctors understand what the limits of gastric banding are.
If you are a health care professional or provider, you are expected by law to educate patients about the risks. Any Lap-Band promotion must clearly include an explanation of the risks.
Any individual with a problem, or who believes there might be a problem with a gastric banding device is encouraged by the FDA to file a voluntary report. This can be done through MedWatch, The FDA Safety Information and Adverse Event Reporting program. The FDA finds these reports extremely helpful when identifying and understanding the risks linked to specific medical devices.
Written by Christian Nordqvist