The sensor augmented Threshold Suspend insulin pump safely reduces nocturnal hypoglycemia without affecting HbA1C (glycated hemoglobin level), says a study published in NEJM (New England Journal of Medicine) and presented at the American Diabetes Association 73rd Scientific Sessions in Chicago, Illinois.
The Threshold Suspend has an investigational MiniMed® integrated system which automatically briefly halts insulin delivery when sensor glucose values hit a pre-set low level, says Medtronic Inc., the makers of the device.
Put simply – Medtronic has designed an insulin pump that can temporarily shot off, thus reducing the risk of nocturnal hypoglycemia, a potentially fatal condition that occurs when a type 1 diabetes patient is asleep and blood glucose levels fall too low. Type 1 diabetes patients must have insulin delivered, either by injections or a pump.
According to Medtronic, this is a major step towards developing a completely automatic artificial pancreas for patients living with diabetes.
Richard M. Bergenstal, M.D., executive director of the International Diabetes Center at Park Nicollet Health Services, said:
“Hypoglycemia can be catastrophic for people with diabetes, especially at night when patients are likely to be unaware of symptoms because they are asleep. These data are very important because they provide strong evidence that sensor-augmented insulin pump therapy with a Threshold Suspend feature can reduce hypoglycemia at home – and it can do it safely, without increasing the patients’ risk for long-term complications by raising their A1C.”
The ASPIRE In-Home study is under an IDE (investigational device exemption) granted by the Food and Drug Administration. It compared two MiniMed sensor-augmented insulin pumps (integrated insulin pumps with continuous glucose monitoring):
- A pump with the Threshold Suspend feature
- A pump without the Threshold Suspend feature
Previous studies have shown that a MiniMed intergrated insulin pump with continuous glucose monitoring provides superior glucose control with no higher risk of hypoglycemia, than taking several injections each day. However, this is the first major in-home study to demonstrate the results of the integrated system which incorporates the Threshold Suspend feature.
Francine Kaufman, M.D., vice president of global medical affairs of the Diabetes business at Medtronic, said:
“ASPIRE In-Home met both its safety and efficacy endpoints and it provides additional clinical validation for Threshold Suspend, the first diabetes technology to automatically take action based on sensor glucose values. The study results are important as we continue to move toward our goal of developing a fully automated system, or artificial pancreas, that will one day require very minimal interaction from the patient.”
The study included 247 type 1 diabetes patients. They all suffered from nocturnal hypoglycemia. Nocturnal hypoglycemia means low blood glucose levels while the patient is asleep.
The participants were given sensor-augmented insulin-pump therapy for three months, and were randomly selected into two groups:
- The Threshold Suspend group
- The non-Threshold Suspend group
The study’s primary safety outcome was alterations in HbA1C. HbA1C is a measurement of a patient’s average blood glucose control over a 3-month period.
The study’s primary efficacy outcome was the AUC (area under the curve) for nocturnal hypoglycemic events. AUC summaries the duration and the magnitude of hypoglycemic events.
The study showed that:
- In the Threshold Suspend Group, the mean AUC for nocturnal hypoglycemic events was 37.5% lower
- In the Threshold Suspend Group, the mean AUC for daytime/nighttime combined hypoglycemic events was 31.4% lower
- HbA1C did not change in either group
- In the Threshold Suspend Group no serious adverse events occurred
The authors of the NEJM report concluded:
“This study showed that over a 3-month period the use of sensor-augmented insulin-pump therapy with the threshold-suspend feature reduced nocturnal hypoglycemia, without increasing glycated hemoglobin values.”
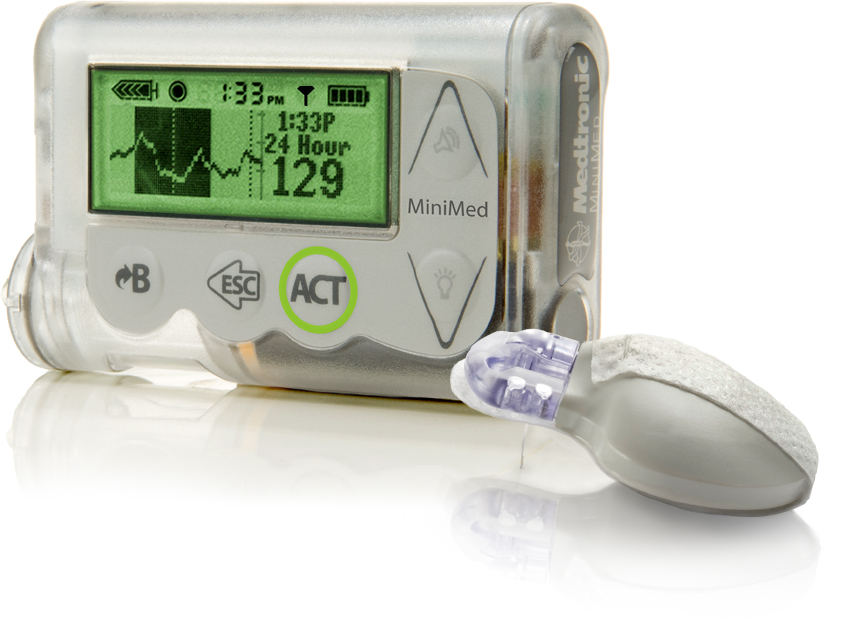
MiniMed integrated system
The Threshold Suspend feature suspends insulin delivery for up to 120 minutes when a patient’s sensor glucose value drops to a preset level. Medtronic says the Threshold Suspend feature, which is exclusive to MiniMed insulin pumps, is a first-of-its-kind.
As soon as the preset low threshold is met, the insulin pump alarms and suspends all insulin delivery for two hours. During this time patients can, whenever they like, resume insulin delivery.
Medtronic wrote “Threshold Suspend is also a part of the MiniMed 530G, which is not commercially available in the U.S. at this time, and is currently under review at the FDA. The MiniMed 530G system is the first system submitted for approval under the new product classification – OZO: Artificial Pancreas Device System, Threshold Suspend – created by the U.S. Food and Drug Administration.”
Medtronic already sells insulin pumps with the automatic temporary shut-off feature in over 50 countries outside the USA.
There are approximately 3 million Americans with type 1 diabetes, which occurs when the person’s immune system destroys insulin-producing beta cells in the pancreas.
Type 1 diabetes patients have to monitor their blood glucose levels and take insulin several times a day.
Dangers of hyperglycemia – if their insulin levels are too low, blood glucose levels shoot up (hyperglycemia), raising the risk of eye damage, kidney failure and heart disease.
Dangers of hypoglycemia – on the other hand, too much insulin pushes blood glucose levels too low (hypoglycemia), raising the risk of seizures, unconsciousness, brain damage and death.
The FDA will not approve insulin pumps with an automatic shut-off feature unless they can examine a large, carefully controlled trial that demonstrates their safety.
The ASPIRE In-Home study was funded by Medtronic MiniMed – ASPIRE ClinicalTrials.gov number NCT01497938.
Written by Christian Nordqvist