Mydayis is a brand-name prescription medication. It’s used to treat attention deficit hyperactivity disorder (ADHD) in adults as well as children ages 13 years and older. ADHD is a mental health condition that can cause problems such as having trouble paying attention or sitting still.
Mydayis belongs to a class of medications called stimulants. (A class of drugs is a group of medications that work in a similar way.) Mydayis contains four forms of the active drug amphetamine:
- dextroamphetamine sulfate
- dextroamphetamine saccharate
- amphetamine aspartate monohydrate
- amphetamine sulfate
The combination of these forms is called mixed amphetamine salts.
Mydayis comes in an extended-release capsule that you take by mouth. (Extended-release drugs enter your bloodstream slowly over time so that you have a steady level of the drug in your body all day.)
Is Mydayis a controlled substance?
Yes, Mydayis is a controlled substance. This means you can become dependent on Mydayis and abuse or misuse it.
The Drug Enforcement Administration (DEA) has created regulations that control how certain drugs are prescribed and how they’re dispensed at a pharmacy. These regulations require you to get a new prescription for each refill. They also limit which type of doctor can write your prescription and how much of the medication is stored in a pharmacy at a given time.
Effectiveness
In five clinical studies, Mydayis was found to ease ADHD symptoms when compared to a placebo (no treatment).
In each of the studies, people with ADHD were given either Mydayis or a placebo. (Each study involved adults as well as children ages 13 years and older.) Everybody was assessed with ADHD rating scales in the beginning of each study, as well as throughout, to measure their ADHD symptoms. At the end of each study, ADHD symptoms had eased significantly in the people who took Mydayis compared to people who took a placebo.
Mydayis is available only as a brand-name medication. It’s not currently available in generic form.
Mydayis contains four forms of the active drug amphetamine:
- dextroamphetamine sulfate
- dextroamphetamine saccharate
- amphetamine aspartate monohydrate
- amphetamine sulfate
The combination of these forms is called mixed amphetamine salts.
The Mydayis dosage your doctor prescribes will depend on several factors. These include:
- the severity of the condition you’re using Mydayis to treat
- your age
- other medications you may be taking
- other medical conditions you may have
Typically, your doctor will start you on a low dosage. Then they’ll adjust it over time to reach the amount that’s right for you. Your doctor will ultimately prescribe the smallest dosage that provides the desired effect.
The following information describes dosages that are commonly used or recommended. However, be sure to take the dosage your doctor prescribes for you. Your doctor will determine the best dosage to suit your needs.
Drug forms and strengths
Mydayis comes as an extended-release capsule. (Extended-release drugs enter your bloodstream slowly over time.) You can take Mydayis by swallowing the capsule. Or you can open the capsule and pour the drug on a spoonful of applesauce, which you then swallow without chewing right away.
Mydayis contains four forms of the active drug amphetamine:
- dextroamphetamine sulfate
- dextroamphetamine saccharate
- amphetamine aspartate monohydrate
- amphetamine sulfate
The combination of these forms is called mixed amphetamine salts.
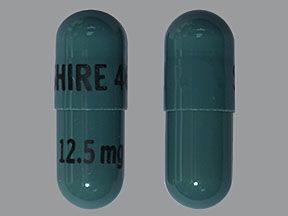
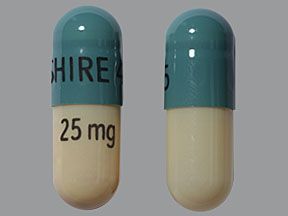
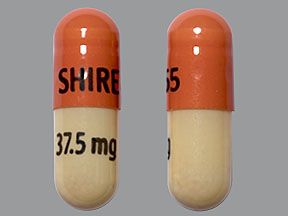
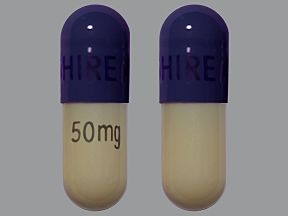
Mydayis is available in four strengths: 12.5 mg, 25 mg, 37.5 mg, and 50 mg. Each strength contains equal amounts of each form of the active amphetamine salts.
Dosage for attention deficit hyperactivity disorder (ADHD)
For adults, you’ll take a 12.5-mg capsule every day. If needed, your doctor may increase your dose by 12.5 mg each week. The maximum dose of Mydayis that you’d take is 50 mg once a day.
Pediatric dosage
Children will take a 12.5-mg capsule every day. If needed, their doctor may increase their dose by 12.5 mg after at least 1 week. The maximum dose of Mydayis that a child would take would be 25 mg once a day.
What if I miss a dose?
You’ll take Mydayis when you first wake up in the morning. If you miss a dose, wait until your next regular dose. Don’t take the missed dose later in the day, and don’t take more than one dose at a time. Avoid taking Mydayis too close to bedtime, as you may have trouble falling asleep.
To help make sure that you don’t miss a dose, put a reminder on your phone. You can also try using a medication timer.
Will I need to use this drug long term?
Mydayis is meant to be used as a long-term treatment.* If you and your doctor determine that Mydayis is safe and effective for you, you’ll likely take it long term.
* Mydayis has a
Other drugs are available that can treat attention deficit hyperactivity disorder (ADHD). Some may be better suited for you than others. If you’re interested in finding an alternative to Mydayis, talk with your doctor. They can tell you about other medications that may work well for you.
Note: Some of the drugs listed here are used off-label to treat these specific conditions. Off-label use is when a drug that’s approved to treat one condition is used to treat a different condition.
Examples of other drugs that may be used to treat ADHD include:
- amphetamine (Adderall XR)
- lisdexamfetamine dimesylate (Vyvanse)
- methylphenidate (Concerta, Focalin XR, Ritalin)
- Atomoxetine hydrochloride (Strattera)
You may wonder how Mydayis compares to other medications that are prescribed for similar uses. Here we look at how Mydayis and Adderall XR (the only available form of Adderall) are alike and different.
Uses
Mydayis and Adderall XR are both prescription medications that belong to a drug class called stimulants. (A class of drugs is a group of medications that work in a similar way.) Stimulants speed up the level of activity in your body’s systems, including your brain, to help make you more alert.
The Food and Drug Administration (FDA) has approved Mydayis to treat attention deficit hyperactivity disorder (ADHD) in adults and children ages 13 years and older.
Adderall XR, an extended-release medication, is approved to treat ADHD in adults as well as children ages 6 years and older.
(The immediate-release form of Adderall is now only available as a generic. This generic form is approved to treat ADHD in adults as well as children ages 3 years and older. It’s also approved to treat narcolepsy in adults as well as children ages 6 years and older. Narcolepsy is a type of sleep problem.)
Both Mydayis and Adderall XR contain four forms of the active drug amphetamine:
- dextroamphetamine sulfate
- dextroamphetamine saccharate
- amphetamine aspartate monohydrate
- amphetamine sulfate
The combination of these forms is called mixed amphetamine salts. Mydayis and Adderall XR are manufactured in different ways to release the active drug over time. They also come in different doses. See below.
Drug forms and administration
Here’s some information about the forms of each drug and how you take them.
Mydayis
Mydayis comes as an extended-release capsule. (Extended-release drugs enter your bloodstream slowly over time.) Mydayis is available in four strengths: 12.5 mg, 25 mg, 37.5 mg, and 50 mg.
You can take Mydayis by swallowing the capsule. Or you can open the capsule and pour the drug on a spoonful of applesauce, which you then swallow without chewing right away. Whichever way you take Mydayis, you’ll take your dose once a day when you wake up in the morning.
Adderall XR
Adderall XR is available in two forms:
- an extended-release capsule in these strengths: 5 mg, 10 mg, 15 mg, 20 mg, 25 mg, and 30 mg
- an immediate-release tablet* in these strengths: 5 mg, 7.5 mg, 10 mg, 12.5 mg, 15 mg, 20 mg, and 30 mg
Your dose of Adderall XR will depend on which form you take. If you take the extended-release capsule, you’ll typically take it once a day when you first wake up. If you take the immediate-release tablet, you’ll typically take the first dose when you wake up. Then you’ll take any additional doses every 4 to 6 hours.
* The immediate-release tablet form of Adderall is available only as a generic.
Side effects and risks
Mydayis and Adderall XR both contain stimulant drugs. (Stimulants speed up the level of activity in your body’s systems, including your brain, to help make you more alert.) Therefore, both medications can cause very similar side effects. Below are examples of these side effects.
- Can occur with Mydayis:
- Can occur with Adderall XR:*
- belly pain
- fatigue (lack of energy)
- emotional lability (rapid changing of emotions)
- fever
- headache
- urinary tract infection (UTI)
- Can occur with both Mydayis and Adderall XR:
Serious side effects
These lists contain examples of serious side effects that can occur with Mydayis, with Adderall XR, or with both drugs (when taken individually).
- Can occur with Mydayis:
- few unique serious side effects
- Can occur with Adderall XR:
- blurred vision
- tics (uncontrollable, spasm-like movements)
- Can occur with both Mydayis and Adderall XR:
- cardiovascular (heart and blood vessel) problems including high blood pressure, rapid heartbeat, heart palpitations (feeling of skipped or extra heartbeats), heart attack, and stroke
- new or worsening of existing psychiatric conditions such as depression or bipolar disorder
- risk of stunted growth in children and teenagers
- peripheral vascular disease, including Raynaud’s phenomenon (lack of blood flow to your nose, ears, fingers, or toes)
- seizures or convulsions (a type of seizure)
Effectiveness
Mydayis and Adderall XR are both FDA-approved to treat ADHD.
These drugs haven’t been directly compared in clinical studies, but studies have found both Mydayis and Adderall XR to be effective for treating ADHD in adults and children (in the approved age ranges).
Costs
Mydayis and Adderall are both brand-name drugs. Mydayis isn’t available in a generic form. But there are generics of the immediate- and extended-release versions of Adderall. Brand-name medications usually cost more than generics.
The actual price depends on your insurance plan, your location, and the pharmacy you use.
In addition to Adderall (above), Vyvanse is another drug that has some uses similar to those of Mydayis. Here we look at how Mydayis and Vyvanse are alike and different.
Uses
Mydayis and Vyvanse are both prescription medications that belong to a drug class called stimulants. (A class of drugs is a group of medications that work in a similar way.) Stimulants speed up the level of activity in your body’s systems, including your brain, to help make you more alert.
The Food and Drug Administration (FDA) has approved both Mydayis and Vyvanse to treat attention deficit hyperactivity disorder (ADHD) in adults.The FDA has also approved both drugs for treatment in children — Mydayis for children ages 13 years and older, Vyvanse for children ages 6 years and older. In addition, Vyvanse is also approved to treat binge eating disorder.
Mydayis contains four forms of the active drug amphetamine:
- dextroamphetamine sulfate
- dextroamphetamine saccharate
- amphetamine aspartate monohydrate
- amphetamine sulfate
The combination of these forms is called mixed amphetamine salts.
Vyvanse contains the active drug lisdexamfetamine dimesylate.
Drug forms and administration
Here’s some information about the forms of each drug and how you take them.
Mydayis
Mydayis comes as an extended-release capsule. (Extended-release drugs enter your bloodstream slowly over time.) Mydayis is available in four strengths: 12.5 mg, 25 mg, 37.5 mg, and 50 mg.
You can take Mydayis by swallowing the capsule. Or you can open the capsule and pour the drug on a spoonful of applesauce, which you then swallow without chewing right away. Whichever way you take Mydayis, you’ll take your dose once a day when you wake up in the morning.
Vyvanse
Vyvanse is available in two forms:
- a capsule in these strengths: 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg, and 70 mg
- a chewable tablet in these strengths: 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, and 60 mg
If you take the Vyvanse chewable tablet, you’ll chew it completely, then swallow it. If you take the Vyvanse capsule, you can swallow it. Or you can open the capsule and pour the drug into orange juice, water, or yogurt, which you then consume right away. Whichever form of Vyvanse you use, you’ll take your dose once a day when you wake up in the morning.
Side effects and risks
Mydayis and Vyvanse both contain stimulant drugs. Therefore, both medications can cause very similar side effects. Below are examples of these side effects.
More common side effects
These lists contain examples of more common side effects that can occur with Mydayis, with Vyvanse, or with both drugs (when taken individually).
- Can occur with Mydayis:
- Can occur with Vyvanse:
- mouth pain
- constipation
- vomiting
- sweating
- fever
- tingling in the hands or feet
- muscle twitches or tics
- itching
- urinary tract infection (UTI)
- nightmares
- Can occur with both Mydayis and Vyvanse:
- insomnia (not being able to sleep)
- reduced appetite or weight loss
- diarrhea
- feeling jittery or anxious
- feeling restless
- dizziness
Serious side effects
This list contains examples of serious side effects that can occur with both Mydayis and Vyvanse (when taken individually):
- seizures or convulsions (a type of seizure)
- peripheral vascular disease, including Raynaud’s phenomenon (lack of blood flow to your nose, ears, fingers, or toes)
- heart problems including high blood pressure, rapid heartbeat, heart palpitations (feeling of skipped or extra heartbeats), heart attack, or stroke
- new or worsening of existing psychiatric conditions such as depression or bipolar disorder
- risk of stunted growth in children and teenagers
Effectiveness
Mydayis and Vyvanse are both FDA-approved to treat ADHD.
These drugs haven’t been directly compared in clinical studies, but studies have found both Mydayis and Vyvanse to be effective for treating ADHD in adults and children. Mydayis is used to treat children ages 13 years and older, while Vyvanse is used to treat children ages 6 years and older.
Costs
Mydayis and Vyvanse are both brand-name drugs. There are currently no generic forms of either drug. Brand-name medications usually cost more than generics.
The actual price you’ll pay for either drug will depend on your insurance plan, your location, and the pharmacy you use.
Mydayis can cause mild or serious side effects. The following lists contain some of the key side effects that may occur while taking Mydayis. These lists don’t include all possible side effects.
For more information on the possible side effects of Mydayis, talk with your doctor or pharmacist. They can give you tips on how to deal with any side effects that may be bothersome.
More common side effects
The more common side effects of Mydayis can include:
- feeling jittery or anxious
- feeling restless
- insomnia (not being able to sleep)
- reduced appetite or weight loss
- dry mouth
- diarrhea
- irregular or painful periods
- erectile dysfunction
Most of these side effects may go away within a few days or a couple of weeks. If they’re more severe or don’t go away, talk with your doctor or pharmacist.
Serious side effects
Serious side effects from Mydayis aren’t common, but they can occur. Call your doctor right away if you have serious side effects. Call 911 if your symptoms feel life-threatening or if you think you’re having a medical emergency.
Serious side effects and their symptoms can include the following:
- Depression. For symptoms, see “Depression” in the “Side effect details” section below.
- Heart problems. Symptoms can include:
- high blood pressure
- rapid heartbeat
- heart palpitations (feeling of skipped or extra heartbeats)
- heart attack
- stroke
- Worsening of existing psychiatric conditions such as bipolar disorder. Symptoms can include:
- impaired and delusional thinking
- aggressive behavior
- agitated or irritable behavior
- Peripheral vascular disease, including Raynaud’s phenomenon (lack of blood flow to your nose, ears, fingers, or toes). Symptoms can include:
- cold or blue-colored fingers and toes
- random pain or tingling in the fingers and toes
- Seizures or convulsions (a type of seizure). Symptoms can include:
- temporary confusion
- loss of awareness or consciousness
- jerking movements of the arms and legs
- Risk of stunted growth in children and teenagers.
Side effect details
You may wonder how often certain side effects occur with this drug, or whether certain side effects pertain to it. Here are several side effects this drug may or may not cause.
Allergic reaction
As with most drugs, some people can have an allergic reaction after taking Mydayis. Symptoms of a mild allergic reaction can include:
- skin rash
- itchiness
- flushing (warmth and redness in your skin)
A more severe allergic reaction is rare but possible. Symptoms of a severe allergic reaction can include:
- swelling under your skin, typically in your eyelids, lips, hands, or feet
- swelling of your tongue, mouth, or throat
- trouble breathing
There were no reports of allergic reactions in clinical studies of Mydayis. However, some allergic reactions have been reported with other amphetamines (drugs that are similar to Mydayis). These allergic reactions ranged from mild to serious.
Call your doctor right away if you have a severe allergic reaction to Mydayis. Call 911 if your symptoms feel life-threatening or if you think you’re having a medical emergency.
Weight loss
You may lose weight when taking Mydayis. This can happen because Mydayis can cause a loss of appetite, which may lead you to lose weight. In clinical studies of Mydayis that lasted up to 7 weeks, 9% of adults who took the drug lost weight. This was compared to 0% of adults who took a placebo (no treatment).
In a 4-week clinical study of Mydayis in children, 5% of those who took Mydayis lost weight, compared to 1% of those who took a placebo.
If you’re concerned about weight loss while taking Mydayis, tell your doctor. They can give you nutrition tips to help make sure you’re at a healthy weight.
Depression
You may have depression or depression-like symptoms when taking Mydayis. In clinical studies, 3% of adults who took Mydayis had depression, compared to 0% of the people who took a placebo. Symptoms of depression can include:
- sleeping for long periods of time
- lack of interest in activities
- loss of appetite or increased appetite
If you have any symptoms of depression while taking Mydayis, talk with your doctor. They can see if your medications need to be adjusted or changed.
Nausea
You may have nausea when taking Mydayis. However, in clinical studies, nausea occurred only in teenagers who took the drug and not in adults. In the studies, 8% of teenagers who took Mydayis had nausea, compared with 4% of teenagers who took a placebo.
If you do have nausea during your Mydayis treatment, it should go away in a few weeks. And the feeling may ease if you eat smaller meals. But if your nausea doesn’t go away, or if you also have vomiting, diarrhea, muscle pain, and flushing, tell your doctor. These could be symptoms of a serious condition called serotonin syndrome (high levels of the chemical serotonin).
Headache
You may have headaches when taking Mydayis. In clinical studies of Mydayis, 1% of adults stopped taking the drug because of headaches. It’s not known how many people stopped taking a placebo because of headaches.
You may have headaches when you first start to take Mydayis or if your doctor increases your dose. Know that the headaches should go away within a few weeks. But if the headaches don’t fade, talk with your doctor about possibly switching to another medication.
Insomnia
You may have insomnia when taking Mydayis. In clinical studies, 31% of adults who took Mydayis had insomnia, but only 2% stopped taking the drug because of sleep problems. Of the adults who took a placebo, 8% had insomnia. In a clinical study with children, 8% of those who took Mydayis had insomnia, compared to 3% of those who took a placebo.
To avoid insomnia, it’s recommended that you take Mydayis in the morning as soon as you wake up. Insomnia may go away as you continue to use Mydayis. If your insomnia doesn’t go away, talk with your doctor about possibly switching to another medication.
Weight gain (not a side effect)
Mydayis doesn’t cause weight gain. Many people who take Mydayis actually have decreased appetite, and some people may lose weight because of it. However,
If you’re concerned about weight gain, talk with your doctor. They can give you nutrition and exercise tips to help make sure you’re at a healthy weight.
Side effects in children
The Food and Drug Administration (FDA) has approved Mydayis to treat attention deficit hyperactivity disorder (ADHD) in adults as well as children ages 13 years and older.
The drug isn’t approved for use in children younger than age 13 years. Clinical studies looked at the safety of Mydayis in children ages 6 to 12 years. The results showed that children were more sensitive to Mydayis than teenagers and adults. This means that children can sometimes have more severe side effects when they take lower doses of Mydayis. Side effects seen in children can include:
- insomnia
- reduced appetite
- risk of stunted growth
Children ages 13 years and older who took Mydayis had the following side effects:
- dizziness
- loss of appetite
- weight loss
- feeling mad or irritable
- insomnia
- nausea
- belly pain
If your child takes Mydayis, their doctor will likely monitor their height and weight. This is to make sure your child is growing at the right rate for their age group.
You should avoid drinking alcohol while taking Mydayis. The drug comes in an extended-release capsule that releases Mydayis into your bloodstream slowly over time. In lab tests, alcohol increased the speed at which Mydayis is absorbed. This means that if you drink alcohol, your body may absorb more Mydayis at a faster rate. And this could lead you to have more severe side effects. (See the “Mydayis side effects” section above to learn more.)
If you drink alcohol and are thinking about taking Mydayis, talk with your doctor. They can suggest ways to help you quit or possibly recommend a different medication.
Mydayis can interact with several other medications. It can also interact with certain supplements as well as certain foods.
Different interactions can cause different effects. For instance, some interactions can interfere with how well a drug works. Other interactions can increase the number of side effects or make them more severe.
Mydayis and other medications
Below is a list of medications that can interact with Mydayis. This list doesn’t contain all drugs that may interact with Mydayis.
Before taking Mydayis, talk with your doctor and pharmacist. Tell them about all prescription, over-the-counter, and other drugs you take. Also tell them about any vitamins, herbs, and supplements you use. Sharing this information can help you avoid potential interactions.
If you have questions about drug interactions that may affect you, ask your doctor or pharmacist.
Mydayis and drugs that can increase levels of serotonin
Certain drugs can increase the level of serotonin in your body. (Serotonin is a chemical that your nerve cells make.) Mydayis can also increase serotonin levels. So taking these drugs with Mydayis may cause serotonin syndrome. This is a dangerous condition in which your body has too much serotonin.
Symptoms can include:
- mental status changes such as hallucinations (seeing or hearing things that aren’t real) or delirium (confusion and reduced awareness of your surroundings)
- dizziness
- high fever
- sweating or feeling flush
Examples of drugs that may lead to serotonin syndrome if used with Mydayis include:
- dextromethorphan (Robitussin DM)
- meperidine (Demerol)
- methadone (Dolophine)
- fluoxetine (Prozac)
- paroxetine (Paxil)
- sertraline (Zoloft)
- venlafaxine (Effexor XR)
- tramadol (Ultram)
- trazodone
If you’re taking a drug that can increase levels of serotonin, tell your doctor before you start taking Mydayis. They can see if your medications need to be adjusted or changed.
Mydayis and certain drugs for heartburn or acid reflux
Taking Mydayis with certain drugs used to treat heartburn or acid reflux may cause your body to absorb more Mydayis. This can increase the number of side effects you have or make them more severe. (See the “Mydayis side effects” section above to learn more.)
Examples of heartburn or acid reflux drugs that may interact with Mydayis include:
- omeprazole (Prilosec)
- sodium bicarbonate (Gaviscon)
- pantoprazole (Protonix)
If you’re taking a drug to treat heartburn or acid reflux, tell your doctor before you start using Mydayis. They may recommend another treatment to help ease your digestive problems.
Mydayis and monoamine oxidase inhibitors (MAOIs)
Certain drugs are absolutely contraindicated with Mydayis, meaning they can’t be used together because it’s too dangerous. Drugs that belong to a class called monoamine oxidase inhibitors (MAOIs) can’t be used with Mydayis. (A class of drugs is a group of medications that work in a similar way.)
Taking an MAOI with Mydayis can put you at serious risk for dangerously high blood pressure or serotonin syndrome. In some cases, serotonin syndrome can even be fatal. (For more about serotonin syndrome, see “Mydayis and drugs that can increase levels of serotonin” above.)
Examples of MAOIs that can’t be taken with Mydayis include:
- isocarboxazid (Marplan)
- phenelzine (Nardil)
- selegiline (Emsam)
- tranylcypromine (Parnate)
If you’re using one of these MAOI drugs, talk with your doctor. You’ll have to stop using the MAOI drug for at least 14 days before you start to take Mydayis.
Mydayis and herbs and supplements
You should check with your doctor or pharmacist before using any herbal or supplement products while taking Mydayis.
Mydayis and St. John’s wort
St. John’s wort is an herbal supplement sometimes used for depression. The supplement has been shown to interact with medications that include amphetamine, such as Mydayis. St. John’s wort can increase the level of a chemical called serotonin in your body. Mydayis can also increase your level of serotonin.
As a result, taking St. John’s wort with Mydayis can cause serotonin syndrome. This is a dangerous condition in which your body has too much serotonin. In some cases, serotonin syndrome can even be fatal. (For more about serotonin syndrome, see “Mydayis and drugs that can increase levels of serotonin” above.)
If you’re taking St. John’s wort, tell your doctor before you start using Mydayis. They may recommend that you stop taking St. John’s wort during your Mydayis treatment.
Mydayis and foods
There are no known interactions between Mydayis and food. See “Taking Mydayis with food” in the “How to take Mydayis” section below to learn more.
Here are answers to some frequently asked questions about Mydayis.
Will I have withdrawal symptoms if I stop using Mydayis?
You might. If you stop taking Mydayis abruptly, you may have symptoms of withdrawal. These can include extreme fatigue (lack of energy) and feelings of depression.
Before you stop taking Mydayis, talk with your doctor. They can advise you on how to slowly reduce the dosage you take to help you avoid withdrawal symptoms.
Will Mydayis slow my child’s growth?
It’s not clear. Stimulant drugs such as Mydayis have been shown to reduce the weight and overall rate of growth in children. (Stimulants speed up the level of activity in your body’s systems, including your brain, to help make you more alert.)
In a 4-week clinical study, 5% of children ages 13 to 17 years who took Mydayis lost weight. This was compared to 1% of children who took a placebo (no treatment). The study didn’t last long enough to judge whether Mydayis affected the children’s growth.
If your child is taking Mydayis, their doctor will likely monitor their weight and height. This is to help make sure your child is growing at the same rate as other children their age.
Are there other treatments for ADHD besides medication?
Yes, but research into how effective they are isn’t as extensive as that of medications. According to the
- behavioral training for children, parents, and peers
- behavioral plans and special classroom programs at school
Natural remedies that researchers are studying include herbal medicines such as ginseng, relaxation techniques such as biofeedback and yoga, and essential oils.
If you’re interested in trying an alternative treatment for ADHD, first check with your doctor.
Is there a cure for ADHD?
No, there’s no cure for ADHD. But taking Mydayis may help you better control your actions and impulses.
Keep in mind that ADHD symptoms can change over time. It’s possible that you may need lower doses of medication for ADHD as you get older.
If you have ADHD, talk with your doctor. They can discuss the best treatment options with you.
The Food and Drug Administration (FDA) approves prescription drugs such as Mydayis to treat certain conditions. Mydayis may also be used off-label for other conditions. Off-label use is when a drug that’s approved to treat one condition is used to treat a different condition.
Mydayis for attention deficit hyperactivity disorder (ADHD)
Attention deficit hyperactivity disorder (ADHD) is a mental health condition that causes you to have trouble paying attention or sitting still. ADHD is typically diagnosed in childhood, and it’s chronic, which means that it doesn’t go away. We don’t know exactly what causes ADHD, but genetics and environment are factors.
Mydayis is FDA-approved to treat ADHD in adults as well as children ages 13 years and older.
In five clinical studies, Mydayis was found to ease ADHD symptoms when compared to a placebo (no treatment). In each of the studies, people with ADHD were given either Mydayis or a placebo. (Each study included adults as well as children ages 13 years and older.)
Everybody was assessed with ADHD rating scales in the beginning of each study, as well as throughout, to measure their ADHD symptoms. At the end of each study, ADHD symptoms had eased significantly in the people who took Mydayis compared to people who took a placebo.
Off-label use for Mydayis
In addition to the use listed above, Mydayis may be used off-label for other uses. Off-label drug use is when a drug that’s approved for one use is used for a different one that’s not approved.
Mydayis for narcolepsy
Narcolepsy is a sleep problem that can cause you to be extremely drowsy during the day and have sudden attacks of sleep. Stimulant medications such as Mydayis are used off-label to treat narcolepsy. Stimulant drugs treat narcolepsy by helping reduce daytime sleepiness. Other stimulant medications that are used for the treatment of narcolepsy include:
- amphetamine salts (Adderall XR)
- dextroamphetamine (Dexedrine)
- methylphenidate (Ritalin)
- dexmethylphenidate (Focalin)
- lisdexamfetamine (Vyvanse)
Mydayis for children
Mydayis is FDA-approved to treat ADHD in children ages 13 years and older.
In five clinical studies, Mydayis was found to ease ADHD symptoms when compared to a placebo (no treatment). In each of the studies, people with ADHD were given either Mydayis or a placebo. (Each study included adults as well as children ages 13 years and older.)
Everybody was assessed with ADHD rating scales in the beginning of each study, as well as throughout, to measure their ADHD symptoms. At the end of each study, ADHD symptoms had eased significantly in the people who took Mydayis compared to people who took a placebo.
As with all medications, the cost of Mydayis can vary. The actual price you’ll pay will depend on your insurance plan, your location, and the pharmacy you use.
Financial and insurance assistance
If you need financial support to pay for Mydayis, or if you need help understanding your insurance coverage, help is available.
Shire LLC, the manufacturer of Mydayis, offers a savings card that may help lower the cost of Mydayis. For more information and to find out if you’re eligible for support, call 800-583-2037 or visit the program website.
You should take Mydayis according to your doctor or healthcare provider’s instructions.
Mydayis comes as an extended-release capsule. (Extended-release drugs enter your bloodstream slowly over time.) You can take Mydayis by swallowing the capsule. Or you can open the capsule and pour the drug on a spoonful of applesauce, which you then swallow without chewing right away.
When to take
Take Mydayis once a day when you wake up in the morning. To help make sure that you don’t miss a dose, put a reminder on your phone. You can also try using a medication timer.
Taking Mydayis with food
Whether you swallow or open up the Mydayis capsule, you can take the drug with or without food. (Keep in mind that if you open the capsule, you’ll still need to pour the drug on applesauce.) If you take Mydayis with food, then keep taking the drug that way. And if you take Mydayis without food, then keep taking the medication that way.
Can Mydayis be crushed, split, or chewed?
If you take Mydayis by swallowing the capsule, don’t crush, split, or chew it. If you take Mydayis by opening the capsule and pouring the drug on applesauce, swallow the applesauce without chewing.
Attention deficit hyperactivity disorder (ADHD) is a mental health condition in which you’re hyperactive and have trouble focusing and controlling your impulses. You may act before thinking. ADHD can affect both adults and children, and the symptoms of ADHD may change as you get older. Symptoms of ADHD in adults aren’t as extreme as those in children. But trouble focusing can still cause challenges in work and social situations.
Researchers haven’t yet fully figured out how ADHD works in the body and why the condition affects certain people. We do know that certain neurotransmitters are involved. (These are certain chemicals in the brain that send messages throughout the body).
The main neurotransmitters involved in ADHD are chemicals called dopamine and norepinephrine. These chemicals play a big role in everyday emotions and thoughts in all people. But in people with ADHD, there might be an imbalance of the chemicals in the brain.
Mydayis is a type of drug called a stimulant. Stimulants speed up the level of activity in your body’s systems, including your brain, to help make you more alert. Mydayis causes your body to release neurotransmitters such as dopamine and norepinephrine. The release of these two chemicals can help improve nerve communication in the brain. And this can help people with ADHD be more alert and attentive.
How long does it take to work?
When you first start taking Mydayis, it could take about a week before you notice a difference in your symptoms. This is because the drug has to build up in your body for you to feel its full effects.
Mydayis comes in an extended-release capsule, so the drug is released over the entire day. This helps keep a steady amount of Mydayis in your system. The drug starts working in about 1 hour and continues to work all day.
It’s not known if Mydayis is safe to take during pregnancy. Mydayis hasn’t been studied in pregnant women. However, stimulant drugs such as Mydayis have been shown to cause low birth weight, premature delivery, and withdrawal symptoms in newborn babies when the mothers took the drug on a regular basis. Stimulants speed up the level of activity in your body’s systems, including your brain, to help make you more alert.
If you’re pregnant or are planning to become pregnant, talk with your doctor before taking Mydayis. They can discuss the pros and cons of the medication with you.
It’s not known if Mydayis is safe to take during pregnancy. There have been some cases of premature delivery or low birth weight in children whose mothers took drugs similar to Mydayis while pregnant. If you or your sexual partner can become pregnant, talk with your doctor about using birth control while using Mydayis.
You shouldn’t breastfeed while taking Mydayis. The drug has been found in the breast milk of women who were taking Mydayis. It’s possible that children who consume breast milk that contains Mydayis may have certain health problems. These include increases in heart rate and blood pressure, blood vessel problems such as peripheral artery disease, and risk of stunted growth.
If you’re planning to breastfeed your child, talk with your doctor before you start taking Mydayis. They can suggest the best ways to feed your child.
This drug comes with several precautions.
FDA warning: Potential for drug misuse and dependence
This drug has a
Mydayis contains active drugs called amphetamines, which are a type of stimulant. (Stimulants speed up the level of activity in your body’s systems, including your brain, to help make you more alert.)
Amphetamines have the potential to be abused. This may lead to a fast heart rate, high blood pressure, anxiety, aggressive behavior, and in rare cases, early death. And taking amphetamines for a long time can cause you to be dependent on them. Before and during your Mydayis treatment, your doctor will monitor you to be sure that you can safely take Mydayis.
Other warnings
Before taking Mydayis, talk with your doctor about your health history. Mydayis may not be right for you if you have certain medical conditions or other factors affecting your health. These include:
- Allergic reactions. You shouldn’t take Mydayis if you’ve ever had an allergic reaction to the drug or any of its ingredients. If you’re not sure whether you’ve had an allergic reaction, ask your doctor. They may suggest a treatment other than Mydayis.
- Use of monoamine oxidase inhibitors (MAOIs). Taking Mydayis with a type of drug called a monoamine oxidase inhibitor (MAOI) may put you at risk for dangerously high blood pressure. If you’re using an MAOI drug, tell your doctor. You’ll have to stop taking the MAOI drug for at least 14 days before you start to take Mydayis.
- Heart problems. If you have a heart problem, Mydayis may cause other heart problems such as stroke, heart attack, and sudden death. Before you start to take Mydayis, tell your doctor if you have any problems with your heart. They may suggest a medication other than Mydayis.
- Bipolar disorder and other mental health conditions. Mydayis can make existing psychiatric conditions worse. If you have bipolar disorder, Mydayis may cause a new psychotic or manic episode. Before you start to take Mydayis, tell your doctor if you have a mental health condition other than ADHD. They can see which treatments are right for you.
- Seizures. If you have a history of seizures or epilepsy, taking Mydayis may make increase your risk of having a seizure. Before you start to take Mydayis, tell your doctor if you have a seizure disorder or a history of seizures. They may suggest a medication other than Mydayis.
- Pregnancy. It’s not known if Mydayis is safe to take during pregnancy. For more information, please see the “Mydayis and pregnancy” section above.
- Breastfeeding. You shouldn’t breastfeed while taking Mydayis.For more information, please see the “Mydayis and breastfeeding” section above.
Note: For more information about the potential negative effects of Mydayis, see the “Mydayis side effects” section above.
Using more than the recommended dosage of Mydayis can lead to serious side effects.
Overdose symptoms
Symptoms of an overdose can include:
- feeling jittery or restless
- tremors (shakiness)
- overactive reflexes (excessive or increased reflexes)
- fast breathing
- confusion
- hallucinations (seeing or hearing things that aren’t real)
- panic
- fever
- muscle pain
- increase in your heart rate or heart rhythm problems such as arrhythmia
- high blood pressure or low blood pressure
- nausea or vomiting
- diarrhea
- seizures
What to do in case of overdose
If you think you’ve taken too much of this drug, call your doctor. You can also call the American Association of Poison Control Centers at 800-222-1222 or use their online tool. But if your symptoms are severe, call 911 or go to the nearest emergency room right away.
When you get Mydayis from the pharmacy, the pharmacist will add an expiration date to the label on the bottle. This date is typically 1 year from the date they dispensed the medication.
The expiration date helps guarantee the effectiveness of the medication during this time. The
Storage
How long a medication remains good can depend on many factors, including how and where you store the medication.
Store Mydayis capsules at 68°F to 77°F (20°C to 25°C) in a tightly sealed container away from light. Avoid storing this medication in areas where it could get damp or wet, such as in bathrooms.
Disposal
If you no longer need to take Mydayis and have leftover medication, it’s important to dispose of it safely. This helps prevent others, including children and pets, from taking the drug by accident. It also helps keep the drug from harming the environment.
The
The following information is provided for clinicians and other healthcare professionals.
Indications
Mydayis is indicated for the treatment of attention deficit hyperactivity disorder (ADHD) in adults as well as children ages 13 years and older.
Mechanism of action
Mydayis contains several amphetamine salt forms: dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine aspartate monohydrate, and amphetamine sulfate, which are amphetamines that work on the sympathomimetic central nervous system.
They increase the following neurotransmitters in the extraneuronal space: dopamine and norepinephrine via reduction of reuptake and monoamines via increased release from synaptic neurons.
Pharmacokinetics and metabolism
Following oral administration of Mydayis, peak plasma concentration is reached in 7 to 10 hours. The mean plasma half-life is about 10 to 13 hours. Steady state is achieved after 7 to 8 days of daily dosing. Metabolism occurs through the CYP2D6 system, with possibility of population-based variants. Excretion occurs primarily through the renal route. In pharmacokinetic studies, approximately half of the given amphetamine was found present in the urine.
Contraindications
Mydayis is contraindicated in patients with known hypersensitivity to Mydayis, or any of its components, including amphetamine. Mydayis is also contraindicated in patients taking monoamine oxidase inhibitors (MAOIs). If a patient is taking an MAOI, they must wait a minimum of 14 days before starting Mydayis. Starting prior to the 14-day window could potentially trigger a hypertensive crisis.
Misuse and dependence
Mydayis has a high potential for abuse and dependence. Each patient should be assessed for their individual risk as it relates to abuse and dependence potential prior to starting Mydayis. While a patient is on Mydayis, it’s important to monitor them for signs of abuse and dependence.
Storage
Mydayis should be stored away from light at room temperature 68°F to 77°F (20°C to 25°C).
Disclaimer: Medical News Today has made every effort to make certain that all information is factually correct, comprehensive, and up-to-date. However, this article should not be used as a substitute for the knowledge and expertise of a licensed healthcare professional. You should always consult your doctor or other healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.