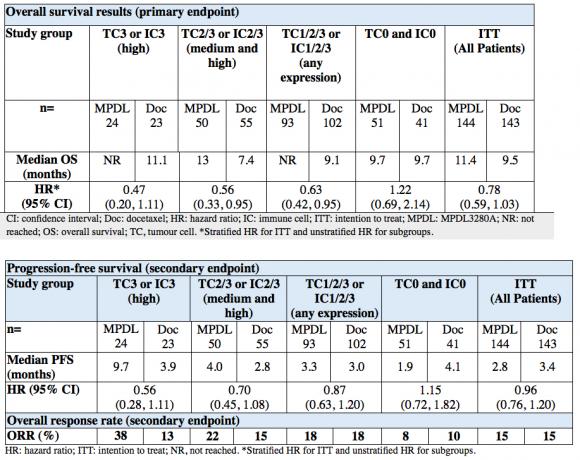
Data to be presented at the 51st Annual Meeting of the American Society of Clinical Oncology (ASCO) suggest that the investigational immunotherapy MPDL3280A may double the likelihood of overall survival in the study period (HR=0.47), compared with docetaxel chemotherapy, in individuals with non-small cell lung cancer (NSCLC) who expressed the highest levels of PD-L1 (a receptor present on cancer cells).1 This Phase II study (POPLAR) in people with previously treated NSCLC is the first trial of its kind to show that inhibiting PD-L1 increases the activity of the immune system which prevents disease progression and may improve survival.1
Lung cancer is Britain's biggest cancer killer, responsible for 6% of all deaths in the UK.2 With around 43,500 new cases diagnosed every year,3 less than 30% of UK lung cancer patients will be alive one year after diagnosis.4 POPLAR, a randomised, multi-centre, global study in NSCLC included patients from The Royal Free Hospital, Charing Cross Hospital and Guy's and St Thomas' Hospital in London, and The Christie in Manchester.1
Through immunohistochemistry testing (IHC), researchers were able to identify individuals with a medium or high expression of PD-L1 on tumour cells and who appear to be most likely to experience improvement in overall survival, progression free survival and overall response rate.1 Conversely this also enabled researchers to identify those who were unlikely to benefit versus standard of care due to low PD-L1 expression.
An improvement in survival was also observed in people who had medium and high (HR=0.56) or any level of PD-L1 expression (HR=0.63), as characterised by the IHC test being developed by Roche,1 demonstrating that this treatment option could improve survival of any individual if they have some PD-L1 cell expression.1
There were no unexpected toxicities experienced with MPDL3280A. It was generally well-tolerated and adverse events were consistent with what has been previously reported for MPDL3280A in NSCLC.1 Roche has a further three Phase II and six Phase III studies of MPDL3280A ongoing in various types of lung cancer. MPDL3280A has shown potential in other areas of unmet need including urothelial bladder cancer of which Phase Ia data is being presented at ASCO.5
Reinforcing Roche's commitment to lung cancer, Roche also presented positive results from two pivotal studies for alectinib, an oral investigational anaplastic lymphoma kinase (ALK) inhibitor which has been shown to shrink tumours (overall response rate, ORR: 50% and 47.8%, respectively) in people with advanced ALK positive (ALK+) NSCLC, whose disease had progressed following treatment with crizotinib.6, 7
Brain metastasis is highly prevalent in patients with ALK+ NSCLC.8 Importantly, these studies show that alectinib shrunk tumours in the central nervous system (CNS) (CNS ORR: 57.1% and 68.8%, respectively) and is therefore able to pass through the blood-brain barrier, the body's natural filter and defence to protect the brain.6, 7 In addition, patients whose tumours had shrunk with alectinib were continuing to respond for a median of 11.2 and 7.5 months respectively.
Alectinib demonstrated a safety profile that was consistent with that observed in previous studies. The most commonly reported adverse events included an increase in muscle enzymes (increased blood levels of creatinine phosphokinase), increased liver enzymes and shortness of breath.6, 7
Both investigational drugs MPDL3280A and alectinib have received Breakthrough Therapy Designation from the FDA - an initiative designed to accelerate the development and review of medicines intended to treat serious diseases and drive patient access through FDA approval.
The European regulatory filings for these indications are still at an early stage as the pivotal trials are currently ongoing. Once the data is available, Roche will liaise with the appropriate health authorities to ensure that patients and doctors receive this therapeutic option as soon as possible.
About non-small cell lung cancer (NSCLC)
Lung cancer is Britain's biggest cancer killer, responsible for 6% of all deaths in the UK.2 Over 43,500 new cases of lung cancer are diagnosed in the UK every year3, resulting in over 35,000 deaths in 2011 alone2 - equivalent to one death every 15 minutes. The prognosis for lung cancer patients is often poor. Research shows that in the UK, only 8.8% of lung cancer patients will be alive five years after diagnosis.4
Lung cancer can be broadly divided into two major types, NSCLC and small cell lung cancer. NSCLC is the most prevalent type, accounting for around 85% of all cases.
About MPDL3280A
MPDL3280A (also known as anti-PDL1 and RG7446) is an investigational monoclonal antibody designed to interfere with a protein called PD-L1. MPDL3280A is designed to target PD-L1 expressed on tumour cells and tumour-infiltrating immune cells, preventing it from binding to PD-L1 and B7.1 on the surface of T cells. By inhibiting PD-L1, MPDL3280A may enable the activation of T cells, restoring their ability to effectively detect and attack tumour cells.
Roche is discussing the interim data from POPLAR with the FDA as part of Breakthrough Therapy Designation in lung cancer. MPDL3280A was granted this in February 2015, for the treatment of people whose NSCLC expresses PD-L1 and who progressed during or after standard treatments (e.g. platinum-based chemotherapy and appropriate targeted therapy for EGFR mutation-positive or ALK-positive disease).
About the POPLAR study
Interim results of the POPLAR study will be presented by Alexander I. Spira, M.D., Ph.D., F.A.C.P, Virginia Cancer Specialists Research Institute; U.S. Oncology Research (Abstract #8010) on Sunday, May 31, 4:42 - 5:54 P.M. CDT. Efficacy, safety and predictive biomarker results from a randomised phase II study comparing MPDL3280A vs docetaxel in 2L/3L NSCLC (POPLAR).1
The Phase II study enrolled 287 patients with previously treated, advanced NSCLC. The primary endpoint was overall survival (OS); secondary endpoints included progression-free survival (PFS), overall response rate (ORR) and safety. Patients were stratified by PD-L1 expression on tumour-infiltrating immune cells (IC), histology and prior lines of therapy. PD-L1 expression was assessed on both tumour cells (TC) and IC; and patients were scored as TC 0, 1, 2, or 3 and IC 0, 1, 2, or 3 with an immunohistochemistry (IHC) test.
Fewer people receiving MPDL3280A experienced Grade 3 to 5 adverse events compared to docetaxel (44 percent vs. 56 percent). More respiratory events were reported for MPDL3280A. The median length of treatment with MPDL3280A was 3.7 months compared to 2.1 months for chemotherapy. Other immune related adverse events in the MPDL3280A arm included increase of enzyme levels in the blood (asparate and alanine aminotransferase; 4% each), inflammation in the lining of the colon (colitis; 1%), inflammation of the liver (hepatitis; 1%) and lung tissue (pneumonitis; 2%).
About Alectinib
Alectinib (RG7853/AF-802/RO5424802/CH5424802) is an investigational oral medicine created at Chugai Kamakura Research Laboratories, part of the Roche Group, and is being developed for people with NSCLC whose tumours are identified as ALK+.6, 7 ALK+ NSCLC is often found in younger people who have a light or non-smoking history. It is almost always found in people with a specific type of NSCLC called adenocarcinoma.
Early studies with alectinib have shown activity on brain metastases, indicating that the drug may be taken up in the brain.6, 7 The brain is protected by the blood-brain barrier, a network of tightly joined cells that line the inside of the blood vessels in the brain and spinal cord. One of the ways the blood-brain barrier prevents molecules from affecting the brain is to actively eject them from the barrier through a process known as 'active efflux'. The active efflux system does not recognise alectinib, which means that it may travel into and throughout brain tissue.
The Global Phase III studies of alectinib include a companion test developed by Roche. Alectinib is marketed in Japan by Chugai Pharmaceutical, a member of the Roche Group.
Alectinib was granted a Breakthrough Therapy Designation from, the FDA in June 2013, for people with ALK+ NSCLC whose disease progressed on crizotinib.
About NP286736
NP28673 is a phase I/II global, single arm, open-label, multicentre trial evaluating the safety and efficacy of alectinib in 138 people with ALK+ NSCLC whose disease progressed on crizotinib.
The study showed by assessment of an independent review committee an ORR in 50.0% of people treated with alectinib, as measured by RECIST criteria.
- An investigator assessment also showed tumours shrank in 47.8% of people who received alectinib.
- CNS tumours shrank in response to alectinib in 57.1% of people whose disease had spread to the brain or other parts of the CNS.
- In addition, the people whose tumours shrank in response to alectinib continued to respond for a median of 11.2 months (DOR, immature data).
- The median progression-free survival (PFS) for people who received alectinib was 8.9 months.
Alectinib demonstrated a safety profile consistent with that observed in previous studies.
- The most common grade 3 or higher adverse event was shortness of breath (dyspnea; 4%).
About NP287617
NP28761 is a phase I/II North American, single arm, open-label, multicentre trial evaluating the safety and efficacy of alectinib in 87 people with ALK+ NSCLC whose disease progressed on crizotinib.
The study showed by assessment of an independent review committee an ORR in 47.8% of people treated with alectinib, as measured by RECIST criteria.
- An investigator assessment showed tumours shrank in 46.0% of people who received alectinib.
- CNS tumours shrank in response to alectinib in 68.8% of people whose disease had spread to the brain or other parts of the CNS.
- In addition, the people whose tumours shrank in response to alectinib continued to respond for a median of 7.5 months (DOR, immature data).
- The immature median PFS was 6.3 months (95% confidence interval [CI] 5.5 - not estimable).
Alectinib demonstrated a safety profile consistent with that observed in previous studies.
- The most common (occurring in at least 2% of people) Grade 3 or higher adverse events were an increase in muscle enzymes (increased blood levels of creatine phosphokinase; 8%), increased liver enzymes (alanine aminotransferase; 6%, and aspartate aminotransferase; 5%) and shortness of breath (dyspnea; 3%).
Adverse reaction reporting
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Roche is committed to the safety of its medicines. As such Roche encourages Adverse Events to be reported and hence the inclusion of this explanatory information. Reporting forms and information can be found at http://www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Roche Products Limited. Please contact Roche Drug Safety Centre by emailing welwyn.uk_dsc@roche.com or calling 01707 367554.