Of the two species of Plasmodium parasites commonly infecting humans, P. vivax grows exclusively in immature red blood cells called reticulocytes. P. falciparum can infect reticulocytes, but it grows primarily in mature red blood cells (called erythrocytes) which make up 99% of red cells in circulation. A study published on June 4th in PLOS Pathogens shows that the different metabolic states of these human host cells provide different growth conditions for the respective parasites - and warn that, as a consequence, drugs that work against one Plasmodium species might fail to be effective against the other.
After their birth in the bone marrow, red blood cells undergo a number of changes to develop into highly specialized oxygen transporters. They expel their nucleus (with its DNA content) before they are released into the blood as reticulocytes. As they mature they get rid of many of their other organelles as well, until they are disk-shaped cells full of hemoglobin, a red protein (which gives blood its color) designed to carry oxygen.
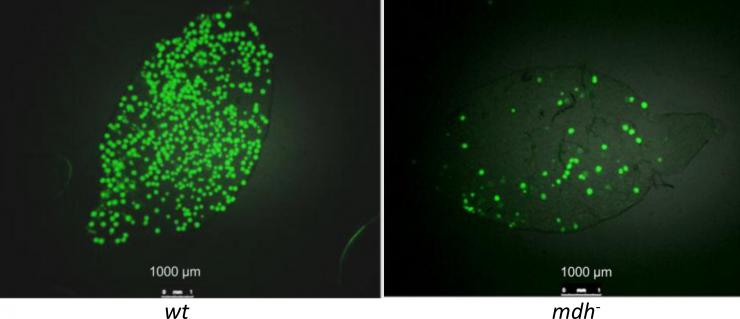
Mosquito mid-guts infected with P. berghei oocysts. Left panel shows a mosquito mid-gut infected with wild-type parasites. Right panel shows a mosquito mid-gut infected with mutant parasites lacking malate dehydrogenase, which make fewer oocysts but still are able to transmit the parasite. Credit: Waters et al., CC-BY
To address whether the two classes of host red blood cells offer different resources for parasite survival, and whether these resources could influence antimalarial drug efficacy, Andy Waters, from the Wellcome Trust Centre for Molecular Parasitology at the University of Glasgow, UK, and colleagues from Glasgow and Melbourne, Australia, undertook a comprehensive biochemical analysis of the metabolites present in reticulocytes on one hand and in mature erythrocytes on the other.
They found that reticulocytes contain elevated levels of many metabolites that could potentially be scavenged by the invading and growing malaria parasite. They also saw a marked overlap in metabolic pathways observed in the reticulocyte and those predicted in the parasite. Such common pathways might be uniquely dispensable to Plasmodium during its growth in reticulocytes while they are essential - and hence a good drug target - for growth in erythrocytes.
To test this hypothesis, the researchers used genetic tools to disrupt some of the overlapping pathways in P. berghei, a species that causes malaria in mice and, similar to P. vivax, has a strong preference for growth in host reticulocytes. They found that indeed such mutant P. berghei strains could grow in mouse reticulocytes (utilizing the host's metabolic products).
Moreover, when the researchers compared the sensitivity to a drug known to target one of the overlapping pathways in a test-tube experiment, they found that P. berghei was considerably less sensitive to the drug than P. falciparum, presumably because the former was able to scavenge the metabolites from their reticulocyte host environment whereas no such external sources were available in the erythrocyte host cells invaded by P. falciparum.
Their data, the researchers say, show that reticulocytes provide a highly enriched host cell environment for Plasmodium parasites. They suggest that "the availability of the reticulocyte metabolome might reduce or block the efficacy of antimalarial drugs that target parasite metabolism. Furthermore reticulocyte resident P. falciparum may enjoy similar protection, giving rise to the possibility that infections could re-emerge."