Researchers have discovered atomic-scale views of an eye protein that maintains transparency of the lens in the human eye, which could potentially lead to new ways of treating and preventing cataracts.
A study, published in the Nature journal Structural and Molecular Biology, shows how scientists have achieved dynamic views of a protein found only in the lenses of mammalian eyes – aquaporin zero (AQP0).
Researchers from the University of California-Irvine and the Janelia Farm Research Campus in Ashurn, VA, explain that the lens in the human eye is made up of unique cells called lens fibers that contain water and proteins called crystallins.
When the fibers and the crystallins are tightly packed, this creates a “uniform medium” that lets light go through the lens. The scientists say that when abnormal development or age-related changes occur in the lens, this can lead to a cataract.
The researchers note that if the AQP0 gene becomes mutated, this can be a cause of congenital cataracts (from birth) and could increase the risk of age-related cataracts.
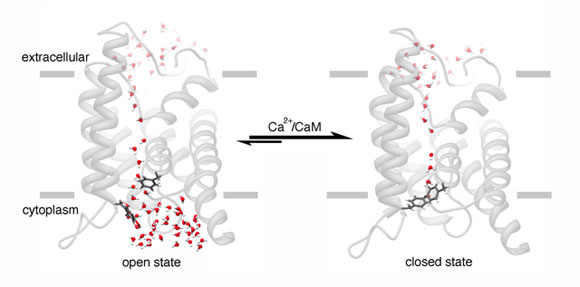
When AQP0 channels close, this is regulated by calmodulin – a calcium sensitive protein. The scientists say that the process of how calmodulin does this was previously unclear, but this dynamic view of the AQP0 channel reveals a process wherein the protein “grasps” the open channel, forcing it to close.
Dr. Houmam Araj of the National Eye Institute, explains:
“The AQP0 channel is believed to play a vital role in maintaining the transparency of the lens and in regulating water volume in the lens fibers, so understanding the molecular details of how water flows through the channel could lead to a better understanding of cataract.”
To get close-up views of the AQP0 protein and calmodulin bound together, the researchers used electron microscopy. They then combined the microscopy data and previous data gathered using crystallography – a process in which proteins are X-rayed after being crystallized.
This process created computerized models of the way the two proteins interact, and it enabled the researchers to identify the critical building blocks for proteins (amino acids) within AQP0.
Scientists then individually neutralized the amino acids within the AQP0 channel.
The study authors explain the AQP0 channel consists of four “barrel-shaped units” that are bundled next to each other.
The close-up view of the AQP0 channel revealed that calmodulin binds to one unit, then to another, as if “grabbing a pair of reins,” the researchers explain.
They add that this process causes the channel to twist, allowing some amino acids in each unit to drift into the channel’s core, blocking the flow of water.
Cataracts are described as cloudy areas of the lens. They can develop in one or both eyes. Though not usually painful, they can severely affect a person’s vision and even lead to blindness eventually. Cataracts are linked to aging, but other risk factors include smoking, diabetes and genetic factors.
The researchers say that this study, funded by the National Eye Institute – part of the National Institutes of Health – could lead to new approaches for treating cataracts.
Dr. James Hall of the University of California, says that although cataracts can be successfully treated with surgery, these findings “may be a step toward learning how to prevent or delay cataract.”
The researchers note that because this research reveals how calmodulin interacts with various protein channels, it could also lead to new drugs for other common health conditions.
For example, they add, calmodulin helps maintain control of ion channels, the passage that allows ions in and out of cells – essential for nerve cell firing, rhythmic beating of the heart and muscle contraction.