Otezla is a brand-name oral tablet that’s prescribed for use in certain people with plaque psoriasis, psoriatic arthritis, and ulcers. Otezla contains the active drug apremilast and belongs to the disease-modifying antirheumatics drug class.
Otezla is approved by the Food and Drug Administration (FDA) for use in certain adults to treat the following conditions:
- plaque psoriasis
- active (currently causing symptoms) psoriatic arthritis, which is a form of arthritis that can occur in people with psoriasis
- ulcers (mouth sores) in people with Behçet’s disease
Drug details
You’ll find key information about Otezla below.
- Drug form: oral tablet
- Generic available? no
- Prescription required? yes
- Controlled substance? no
- Year of FDA approval: 2014
Otezla contains the drug apremilast. It’s not available as a generic drug. (A generic drug is an exact copy of the active drug in a brand-name medication.) Apremilast is only available as Otezla.
Otezla can cause mild or serious side effects. The following list contains some of the key side effects that may occur while taking Otezla. This list doesn’t include all possible side effects.
For more information on the possible side effects of Otezla, or for tips on how to deal with a troubling side effect, talk with your doctor or pharmacist.
Note: The Food and Drug Administration (FDA) tracks side effects of drugs they have approved. If you would like to report to the FDA a side effect you’ve had with Otezla, you can do so through MedWatch.
More common side effects
The more common side effects of Otezla include:
- diarrhea
- nausea
- headache
- respiratory infection
- vomiting
- abdominal pain
- fatigue
- insomnia
- decreased appetite
- weight loss
- back pain
Most of these effects may go away within a few days or a couple of weeks. If they’re more severe or don’t go away, talk with your doctor or pharmacist.
* This is a partial list of mild side effects from Otezla. To learn about other mild side effects, talk with your doctor or pharmacist, or visit Otezla’s prescribing information.
Serious side effects
Call your doctor right away if you have serious side effects. Call 911 or your local emergency number if your symptoms feel life threatening or if you think you’re having a medical emergency. Serious side effects and their symptoms can include the following:
- allergic reaction*
- severe diarrhea, nausea, or vomiting
- depression
- thoughts of suicide
* Allergic reaction wasn’t reported in clinical trials but has occurred since the drug was approved.
Side effect details
You may wonder how often certain side effects occur with this drug. Here’s some detail on certain side effects this drug may cause. For additional details about side effects, see this article.
Allergic reaction
As with most drugs, some people can have an allergic reaction after taking Otezla. Allergic reactions weren’t reported in clinical trials of Otezla but have occurred since the drug was approved.
Symptoms of a mild allergic reaction can include:
- skin rash
- itchiness
- flushing
A more severe allergic reaction is rare but possible. Symptoms of a severe allergic reaction can include:
- swelling under your skin, typically in your eyelids, lips, hands, or feet
- swelling of your tongue, mouth, or throat
- trouble breathing
Call your doctor right away if you have a severe allergic reaction to Otezla. But call 911 or your local emergency number if your symptoms feel life threatening or if you think you’re having a medical emergency.
Weight loss
Loss of appetite and weight loss are common side effects of Otezla. In most cases, weight loss was minor, but some people have experienced more significant changes in body weight.
If you experience severe weight loss while taking Otezla, talk with your doctor. They may recommend that you stop taking this drug.
Cancer
People who have psoriasis have a slightly increased risk for certain types of cancer. There’s also a concern that some of the medications used for treating psoriasis might increase the risk of some types of cancer.
Clinical studies on apremilast, the drug contained in Otezla, so far show that it doesn’t increase the risk of cancer in people who have psoriasis.
Depression
Although not common, depressed mood can occur in some people who take Otezla. During clinical trials, very few people experience this side effect. And even fewer people experience serious or more severe depression. Suicidal thoughts or behaviors are also uncommon in people taking Otezla.
Depression in people taking Otezla might be more likely for those who have had depression in the past.
If you experience mood changes or depressed mood while taking Otezla, be sure to talk with your doctor.
Suicide prevention
If you know someone at immediate risk of self-harm, suicide, or hurting another person:
- Ask the tough question: “Are you considering suicide?”
- Listen to the person without judgment.
- Call 911 or the local emergency number, or text TALK to 741741 to communicate with a trained crisis counselor.
- Stay with the person until professional help arrives.
- Try to remove any weapons, medications, or other potentially harmful objects.
If you or someone you know is having thoughts of suicide, a prevention hotline can help. The 988 Suicide and Crisis Lifeline is available 24 hours a day at 988. During a crisis, people who are hard of hearing can use their preferred relay service or dial 711 then 988.
Headaches
Headache is a common side effect that people who take Otezla report. It may be more common in certain people taking the drug, depending on the condition being treated.
In most cases, people experience a milder tension-type headache. But some people may experience a migraine headache, which is more severe.
These side effects usually go away with continued use of Otezla. If they don’t go away or become bothersome, talk with your doctor.
Diarrhea
Diarrhea commonly occurs in people who take Otezla. In fact, nearly half of people taking the drug have diarrhea. Most of the time, the diarrhea isn’t severe and usually goes away with continued use of the drug.
However, severe diarrhea has occurred in some people taking Otezla, and in some cases, it can lead to dehydration (low fluid level) and electrolyte imbalances.
If you have diarrhea that doesn’t go away or you have severe diarrhea while taking Otezla, talk with your doctor. They may lower your dosage or have you stop taking the drug.
Nausea
Nausea is a common side effect of Otezla. In most cases, the nausea isn’t severe and usually goes away with continued use of the drug.
However, in some cases, it can be severe and may include vomiting. Severe nausea and vomiting can lead to dehydration (low fluid level) and electrolyte imbalances.
If your nausea doesn’t go away or you have severe nausea or vomiting while taking Otezla, talk with your doctor. Your doctor may lower your dosage or have you stop taking Otezla.
As with all medications, the cost of Otezla can vary. The actual price you’ll pay depends on your insurance plan, your location, and the pharmacy you use.
Before approving coverage for Otezla, your insurance company may require you to get prior authorization. This means that your doctor and insurance company will need to communicate about your prescription before the insurance company will cover the drug. The insurance company will review the prior authorization request and decide if the drug will be covered.
If you’re not sure if you’ll need to get prior authorization for Otezla, contact your insurance company.
Financial and insurance assistance: If you need financial support to pay for Otezla, or if you need help understanding your insurance coverage, help is available.
Amgen, the manufacturer of Otezla, offers a copay card that may reduce the cost of Otezla. The manufacturer also has a program called Amgen SupportPlus, which may assist you in finding ways to lower the cost of Otezla. For more information and to find out if you’re eligible for support, call 844-468-3952 or visit the program website.
Generic version: Otezla is not available in a generic form. A generic drug is an exact copy of the active drug in a brand-name medication. Generics tend to cost less than brand-name drugs.
Drug coupons: Check out coupons and saving options from the drug manufacturer, to save money on your Otezla prescription.
To learn more about saving money on prescriptions, check out this article. And for more information about the cost of Otezla, see this article.
Save on your Otezla prescription
Use your insurance to pay as little as $- through Otezla’s manufacturer savings card.
The Food and Drug Administration (FDA) approves prescription drugs such as Otezla to treat certain conditions.
Otezla is FDA-approved to treat three conditions in certain adults, which are described in more detail below:
- plaque psoriasis
- active (currently causing symptoms) psoriatic arthritis
- mouth ulcers caused by Behçet’s disease
For plaque psoriasis and psoriatic arthritis, Otezla is often used in combination with other medications such as methotrexate (Otrexup, Rasuvo, Trexall, others), sulfasalazine (Azulfidine), leflunomide (Arava), or others.
Otezla for psoriatic arthritis
Otezla is approved to treat active psoriatic arthritis in adults. Psoriatic arthritis is a condition that has both the swollen, sore joints of arthritis and the skin lesions of psoriasis. Skin lesions caused by psoriasis are usually itchy, scaly red patches. In some cases, the lesions can affect your scalp.
For more information about your condition, you can visit our arthritis hub.
Otezla for plaque psoriasis
Otezla is approved to treat plaque psoriasis in certain adults. Specifically, it can be used in adults who are candidates for phototherapy (light therapy) or systemic therapy (treatment that affects the entire body).
Plaque psoriasis is the most common form of psoriasis. It’s characterized by thick red patches of skin that often have a silver or white scaly layer.
For more information about your condition, you can visit our psoriasis hub.
Otezla for mouth ulcers caused by Behçet’s disease
Otezla is approved to treat mouth ulcers that occur with Behçet’s disease.
Behçet’s disease is an autoimmune disease. It causes damage to certain blood vessels that can lead to sores in your mouth, rashes, and other symptoms.
Otezla for other conditions
In addition to the uses listed above, Otezla may be used off-label for other uses. Off-label drug use is when a drug that’s approved for one use is used for a different one that’s not approved. And you may wonder if Otezla is used for certain other conditions. Below is information on other possible uses for Otezla.
Otezla for other forms of psoriasis (not an approved use)
There are several forms of psoriasis, but Otezla is only approved to treat active plaque psoriasis.
However, Otezla is used off-label for adults with guttate psoriasis, nail psoriasis, palmoplantar psoriasis, pustular psoriasis, and scalp psoriasis. It’s not recommended for off-label use in treating erythrodermic psoriasis.
If you have questions about treatment options for psoriasis, talk with your doctor. For more information about your condition, you can also visit our psoriasis hub.
Otezla for eczema (not an approved use)
Eczema, also called atopic dermatitis, can result in long lasting or recurring rashes on the face, head, or arms and legs.
In 2018, one small study evaluated Otezla for treating adults with eczema and found that it reduced itching and the severity of eczema. However, treatment guidelines from the American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology do not currently recommend Otezla for treating eczema.
If you’d like to know about treatment options for eczema, talk with your doctor. For more information about your condition, you can also visit our eczema hub.
Otezla for rheumatoid arthritis (not an approved use)
Currently, the
One
If you’d like to learn about treatment options for RA, talk with your doctor. For more information about your condition, you can also visit our RA hub.
Alcohol is not known to interact with Otezla. However, drinking alcohol, especially in large amounts, while taking Otezla might add to or worsen some side effects from Otezla.
Worsened side effects can include diarrhea, nausea, vomiting, headache, and fatigue.
If you have questions about the safety of drinking alcohol while taking Otezla, talk with your doctor or pharmacist.
Otezla can interact with several medications. It can also interact with certain supplements.
Before taking Otezla, talk with your doctor and pharmacist. Tell them about all prescription, over-the-counter, and other drugs you take. Also tell them about any vitamins, herbs, and supplements you use. Sharing this information can help you avoid potential interactions.
For more information about Otezla and interactions, see this in-depth article.
Otezla and other medications
Below is a list of medications that can interact with Otezla. This list doesn’t contain all drugs that may interact with Otezla.
Different drug interactions can cause different effects. For instance, some can interfere with how well a drug works, while others can cause increased side effects.
Before taking Otezla, be sure to tell your doctor and pharmacist about all prescription, over-the-counter, and other drugs you take. Also tell them about any vitamins, herbs, and supplements you use. Sharing this information can help you avoid potential interactions.
If you have questions about drug interactions that may affect you, ask your doctor or pharmacist.
Drug metabolism inducers
Several medications can make an enzyme (a type of protein) called cytochrome P450 3A4 more active in your body. Taking these drugs with Otezla can cause your body to metabolize (get rid of) Otezla more quickly than usual. It can also make Otezla less effective.
Examples of these medications include:
- carbamazepine (Carbatrol, Epitol, Equetro, Tegretol)
- phenobarbital
- phenytoin (Dilantin, Phenytek)
- primidone (Mysoline)
- rifampin (Rifadin)
Herbs and supplements
Herbs and supplements can sometimes interact with medications.
St. John’s wort
St. John’s wort can make an enzyme called cytochrome P450 3A4 more active in your body. Because of this, taking St. John’s wort with Otezla can cause your body to get rid of Otezla more quickly than usual. This can make Otezla less effective.
Here are answers to some frequently asked questions about Otezla.
Does Otezla cause hair loss?
Hair loss isn’t a side effect that’s been found in clinical studies of Otezla. However, some people have experienced hair loss while taking Otezla. It’s not clear if Otezla is the cause.
Psoriasis, especially scalp psoriasis, can cause hair loss.
Is Otezla an anti-inflammatory drug?
No, Otezla isn’t classified as an anti-inflammatory drug. Although it does reduce inflammation, it doesn’t belong to the class of drugs called anti-inflammatories.
Is Otezla an immunosuppressant?
Yes, Otezla is an immunosuppressant. This means the drug lowers (suppresses) the activity of your immune system. Specifically, Otezla works by reducing inflammation that’s caused by an overactive immune system.
Is Otezla a biologic?
No, Otezla isn’t a biologic. Biologics are drugs that are made from immune system cells. Otezla, however, is made from chemicals (as many drugs are).
Otezla is a type of drug called an immunosuppressant. It works by lowering (suppressing) the activity of your immune system.
To learn about how Otezla compares with biologic drugs, see the “Otezla vs. biologics” section below.
How does Otezla cause weight loss?
Many people who take Otezla lose weight. There may be several factors that lead to Otezla-related weight loss.
Otezla blocks an enzyme called phosphodiesterase-4 (PDE4). In addition to its effects on inflammation, this enzyme is involved in energy metabolism. In animals, blocking this enzyme caused them to be leaner, with smaller fat cells. The same effect may apply in humans.
Also, some people who take Otezla may have a reduced appetite or diarrhea as side effects. These effects might also cause weight loss.
I’ve always used creams for my psoriasis. How does a pill help treat my psoriasis?
Creams and other medications applied to the skin work by being absorbed through the skin. They reduce inflammation and excessive cell growth in the area around where the medication is applied. These drugs are usually the first medications used for psoriasis.
Pills used for psoriasis work from the inside out. They work throughout the body by blocking the body’s production of chemical messengers that cause inflammation and cell overgrowth on the skin.
I’ve heard that Otezla causes a lot of nausea and vomiting. How can I prevent this?
Yes, many people who take Otezla can have some nausea or vomiting. This is most likely to occur in the first 2 weeks of taking the medication. For most people, it’s not severe, and it often goes away with continued use of the drug.
To prevent nausea and vomiting, your doctor may need to lower your dosage. If your nausea doesn’t go away or becomes severe, talk with your doctor. If lowering the dose doesn’t help, you may need to stop taking Otezla.
When you start taking Otezla, your doctor will gradually increase your dosage until you reach the standard dose. Your doctor may follow a specific schedule that the drug manufacturer recommends.
The following information describes the dosages that are commonly used or recommended. However, be sure to take the dosage your doctor prescribes for you.
To learn more about Otezla’s dosages, visit this comprehensive article.
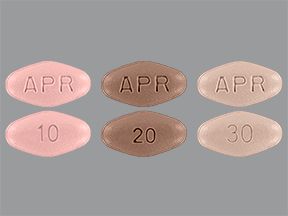
Forms and strengths
Otezla comes as tablets that are taken by mouth. It’s available in the following strengths: 10 milligrams (mg), 20 mg, and 30 mg.
Dosage for psoriatic arthritis, plaque psoriasis, and Behçet’s disease
When you first start taking Otezla, your doctor will likely increase your dosage gradually over 5 days. This is to help reduce gastrointestinal (GI) upset.
The dosing schedule for Otezla is as follows:
| Day of treatment | Dosage |
| Day 1 | Morning: 10 mg |
| Day 2 | Morning: 10 mg Evening: 10 mg |
| Day 3 | Morning: 10 mg Evening: 20 mg |
| Day 4 | Morning: 20 mg Evening: 20 mg |
| Day 5 | Morning: 20 mg Evening: 30 mg |
| Day 6 and after | Morning: 30 mg Evening: 30 mg |
Dosage considerations
If you have kidney disease, your doctor may prescribe a different dosage. During the 5-day starting period, you may only take the morning doses and skip the evening dose. On day 6 and after, your dosage would then be 30 mg once daily.
Your doctor may also prescribe a lower dosage if you’ve experienced troubling side effects such as serious diarrhea, nausea, or vomiting.
What if I miss a dose?
If you miss a dose, take it as soon as you remember. If it’s almost time for your next dose, just take that one dose. Don’t try to catch up by taking two doses at once.
Stopping Otezla doesn’t cause withdrawal symptoms. (Withdrawal symptoms are uncomfortable side effects that can occur when you stop taking a drug your body is used to.) However, you should still talk with your doctor before stopping this medication. And keep in mind that if you do stop taking it, the symptoms of your condition may return.
The manufacturer of Otezla offers information and support for people taking Otezla through a special program. This program, called Amgen SupportPlus, also provides information on how to reduce costs for the drug. You can learn more on the program website or by calling 844-468-3952.
Several types of drugs can be used to treat psoriasis, psoriatic arthritis, and Behçet’s disease, which are the conditions Otezla is approved to treat.
Note: Some of the drugs listed below are used off-label to treat these specific conditions. Off-label use is when a drug that’s approved to treat one condition is used to treat a different condition.
Other DMARDs
Otezla belongs to a class of medications called disease-modifying antirheumatic drugs (DMARDs). Other DMARDs that may be used to treat psoriasis, psoriatic arthritis, or Behçet’s disease include:
- leflunomide (Arava)
- methotrexate (Otrexup, Rasuvo, Trexall, others)
- sulfasalazine (Azulfidine)
Medications from other drug classes
Medications in other drug classes may also be used as alternatives to Otezla for certain diseases. Examples of these drugs include:
- retinoids for psoriasis, psoriatic arthritis, or Behçet’s disease, such as:
- acitretin
- isotretinoin (Absorica, Amnesteem, Claravis, others)
- immunosuppressants for psoriasis, psoriatic arthritis, or Behçet’s disease, such as:
- azathioprine (Azasan, Imuran)
- cyclosporine (Gengraf, Neoral, Sandimmune)
- biologics for psoriasis or psoriatic arthritis, such as:
Herbs and supplements
Some people also use herbs and dietary supplements in an effort to treat psoriasis, psoriatic arthritis, or Behçet’s disease. Examples of these supplements include:
- aloe cream
- fish oil
- saffron
- St. John’s wort ointment
Be sure to talk with your doctor before trying any herb or dietary supplement for treating psoriasis or psoriatic arthritis. For most of these supplements, either there’s very little research showing that they work, or research findings are inconsistent.
You may wonder how certain drugs, such as Humira, compare to Otezla.
Otezla and Humira (adalimumab) belong to different classes of medication. A medication class describes a group of drugs that work in the same way. Otezla is a disease-modifying antirheumatic drug (DMARD). Humira, on the other hand, is a biologic therapy that’s in a class of drugs called tumor necrosis factor-alpha (TNF-alpha) inhibitors.
Use
Both Otezla and Humira are FDA-approved for treating psoriasis and psoriatic arthritis in adults. However, Humira is also FDA-approved to treat many other conditions, including rheumatoid arthritis, Crohn’s disease, ulcerative colitis, and others.
In addition, Otezla is approved to treat mouth sores in adults with Behçet’s disease. Humira is approved to treat certain conditions in children, and Otezla is approved for use in adults only.
Both medications can be taken by themselves or along with other medications for these uses.
Forms and administration
Otezla comes as a tablet that’s typically taken by mouth twice daily.
Humira is a subcutaneous injection that’s given every other week or every week, depending on the condition being treated.
Effectiveness
Both Otezla and Humira are effective for treating psoriasis and psoriatic arthritis. They haven’t been directly compared in clinical studies.
When comparing drugs, keep in mind that your doctor will make treatment recommendations based on your individual needs. They’ll consider several factors, such as your age, other conditions you may have, your risk for side effects, and how severe your condition is.
Side effects and risks
Otezla and Humira have some similar side effects and some that differ. Below are examples of these side effects.
| Both Otezla and Humira | Otezla | Humira | |
| More common side effects | • respiratory infection • headache • nausea • abdominal pain • back pain | • diarrhea • fatigue • decreased appetite • weight loss | • sinusitis • flu-like symptoms • rash • high cholesterol • urinary tract infections • injection-site reactions |
| Serious side effects | • allergic reaction | • severe diarrhea • severe nausea and vomiting • depression • thoughts of suicide | • heart failure • blood disorders • serious infections such as tuberculosis • cancer • nervous system conditions such as multiple sclerosis and Guillain-Barré syndrome • lupus-like syndrome |
Costs
Otezla and Humira are both available only as brand-name drugs. They don’t have generic forms, which are typically less expensive than brand-name versions.
You may wonder how Otezla compares with Enbrel. Otezla contains the active drug apremilast, and Enbrel contains etanercept.
Both drugs are FDA-approved to treat psoriatic arthritis and plaque psoriasis in adults.
In addition, Otezla is approved for use in adults to treat mouth sores caused by Behçet’s disease. And Enbrel is approved to treat certain other forms of arthritis in adults and some children.
See this article for more information about Enbrel. Your doctor or pharmacist can also provide more information about how Otezla and Enbrel compare.
You may wonder how certain drugs, such as Stelara (ustekinumab), compare to Otezla.
Otezla and Stelara belong to different classes of medication. A medication class describes a group of drugs that work in the same way. Otezla is a disease-modifying antirheumatic drug (DMARD). Stelara is a biologic therapy that’s in a class of drugs called interleukin inhibitors.
Use
Both Otezla and Stelara are FDA-approved to treat plaque psoriasis and psoriatic arthritis in adults. Stelara is also approved to treat these conditions in children ages 6 years and older.
In addition, Stelara is also FDA-approved to treat Crohn’s disease and ulcerative colitis in adults. Otezla is also approved to treat mouth sores in adults with Behçet’s disease.
Both medications can be taken by themselves or along with other medications for these uses.
Forms and administration
Otezla comes as a tablet that’s typically taken by mouth twice daily.
Stelara is available as a subcutaneous injection and as a solution that’s given by intravenous (IV) infusion. (With an IV infusion, the drug is injected into your vein over a period of time.) Dosages of Stelara depend on your age, weight, and the condition you’re treating.
Effectiveness
Both Otezla and Stelara are effective for treating plaque psoriasis and psoriatic arthritis. These drugs haven’t been directly compared in clinical studies.
When comparing drugs, keep in mind that your doctor will make treatment choices based on your individual needs. They’ll consider several factors, such as your age, other conditions you may have, your risk of side effects, and how severe your condition is.
Side effects and risks
Otezla and Stelara have some similar side effects and some that differ. Below are examples of these side effects.
| Both Otezla and Stelara | Otezla | Stelara | |
| More common side effects | • respiratory infection • headache • fatigue • diarrhea • back pain • abdominal pain • nausea | • decreased appetite weight loss | • dizziness • itchiness • throat pain |
| Serious side effects | • allergic reaction | • severe diarrhea • severe nausea and vomiting • depression • thoughts of suicide | • serious infection • pneumonia (not caused by infections) • cancer |
Costs
Otezla and Stelara are both only available as brand-name drugs. They don’t have generic forms, which are typically less expensive than brand-name versions.
Taking too much of this medication can increase your risk for serious side effects.
Overdose symptoms
Symptoms of an overdose of Otezla may include:
- severe diarrhea, nausea, and vomiting
- headache
- fatigue
- dizziness
What to do in case of overdose
If you think you’ve taken too much of this drug, call your doctor or seek guidance from America’s Poison Centers at 800-222-1222 or through its online tool. But if your symptoms are severe, call 911 or go to the nearest emergency room right away.
Overdose treatment
Treatment of an overdose will depend on the symptoms that occur. A doctor may order certain tests to monitor for side effects. In some cases, they may even prescribe intravenous (IV) fluids.
There haven’t been enough studies in humans to know if Otezla is safe to use during pregnancy. Studies in animals have shown potential harm to a fetus when Otezla was given to pregnant females. However, animal studies don’t always predict the way humans would respond.
If you’re planning a pregnancy or can become pregnant, talk with your doctor before taking this medication.
Otezla’s pregnancy registry
The manufacturer of Otezla created a registry that gathered information about the safety of Otezla use during pregnancy. The registry is no longer open for enrollment.
The Otezla pregnancy registry recorded health information about parents and babies who may have been affected by Otezla. This information helps doctors and other people using the drug to know more about the safety of Otezla use during pregnancy.
It’s not known if Otezla is safe to take during pregnancy. If you’re sexually active and you or your partner can become pregnant, talk with your doctor about your birth control needs while you’re using Otezla.
For more information about taking Otezla during pregnancy, see the “Otezla and pregnancy” section above.
There haven’t been enough studies to show whether Otezla appears in human breast milk. In animal studies, Otezla was found to be present in breast milk when the drug was given to lactating animals. But animal studies don’t always predict how human studies would go.
Until more is known, your doctor will likely recommend that you should avoid breastfeeding while taking this drug.
Otezla and biologic therapies can both be used to treat psoriasis and psoriatic arthritis. But keep in mind that Otezla isn’t a biologic. (Biologics are drugs that are made from living cells, such as immune system cells.) Otezla, however, is made from chemicals (as many drugs are).
Otezla is a type of drug called an immunosuppressant. It works by lowering (suppressing) the activity of your immune system.
Here are some points to consider when comparing Otezla to biologic drugs:
- Otezla hasn’t been directly compared to biologic therapy in clinical studies.
In some cases , biologic therapy may have more risks in terms of potentially serious side effects.- Biologic drugs are often more expensive than Otezla.
- Otezla is a tablet that you take by mouth. Biologic therapies are all given by injection.
Keep in mind that your doctor will make treatment choices based on your individual needs. They’ll consider several factors, such as your age, other conditions you may have, your risk for side effects, and how severe your condition is.
There are many different types of biologic therapies. Examples of these drugs include:
- tumor necrosis factor-alpha inhibitors, such as:
- interleukin 12 and 23 inhibitors, such as:
- ustekinumab (Stelara)
- interleukin 17 inhibitors, such as:
- interleukin 23 inhibitors, such as:
- guselkumab (Tremfya)
- T-cell inhibitors, such as:
- abatacept (Orencia)
Biologics are medications that can be made from sugars, proteins, or nucleic acids, or from microorganisms, tissues, or cells. Traditional drugs are usually made from chemicals or plants.
Otezla is typically taken twice daily: once in the morning and once in the evening. For some people, such as those with kidney problems, it may be taken just once per day, in the morning.
Otezla can be taken on an empty stomach or with food.
Otezla tablets should be swallowed whole. They shouldn’t be crushed, split, or chewed.
Otezla works in a unique way compared to other medications that are used to treat plaque psoriasis, psoriatic arthritis, or Behçet’s disease. It blocks an enzyme called phosphodiesterase-4 (PDE4), which is found in immune cells.
By blocking this enzyme, Otezla decreases the body’s production of inflammatory molecules. The actions of these molecules can lead to the symptoms of plaque psoriasis, psoriatic arthritis, and Behçet’s disease. Therefore, decreasing their production helps reduce symptoms.
When Otezla is dispensed from the pharmacy, the pharmacist will add an expiration date to the label on the bottle. This date is typically 1 year from the date the medication was dispensed.
The purpose of such expiration dates is to guarantee the effectiveness of the medication during this time.
The
Storage
How long a medication remains good can depend on many factors, including how and where the medication is stored. Otezla should be stored at room temperature, below 86°F (30°C).
Disposal
If you no longer need to take Otezla and have leftover medication, it’s important to dispose of it safely. This helps prevent others, including children and pets, from taking the drug by accident. It also helps keep the drug from harming the environment.
This article provides several useful tips on medication disposal. You can also ask your pharmacist for information on how to dispose of your medication.
Before taking Otezla, talk with your doctor about any medical conditions you have. Otezla may not be appropriate for you if you have certain medical conditions. These include:
- Depression. Depressed mood can occur in some people who take Otezla. Some people experience thoughts of suicide while taking Otezla. Although this isn’t common, it may be more likely in people who have had depression in the past.
- Kidney problems. If you have kidney problems, you may need to take a lower dosage of Otezla.
- Allergic reaction. If you’ve had an allergic reaction while taking Otezla or to any of its ingredients, you should not take Otezla. Your doctor can recommend another drug that can treat your condition.
- Pregnancy. If you’re planning a pregnancy or can become pregnant, talk with your doctor before taking this medication. For more information, see the “Otezla and pregnancy” section above.
- Breastfeeding. If you’re breastfeeding or are planning to do so, talk with your doctor before taking this medication. For more information, see the “Otezla and pregnancy” section above.