Invega Trinza (paliperidone palmitate) is a brand-name prescription medication. The Food and Drug Administration (FDA) has approved it for use in certain adults to treat schizophrenia. They must have previously received Invega Sustenna for 4 months or longer. (Invega Sustenna is another drug used to treat schizophrenia.)
Invega Trinza comes as a liquid suspension in a prefilled syringe. A healthcare professional will give you your doses as intramuscular (IM) injections.
Invega Trinza contains the active drug paliperidone palmitate, which belongs to a class of drugs called atypical antipsychotics.
Invega Trinza is currently available only as a brand-name medication. It does not have a generic form.
Invega Trinza vs. Invega, Invega Sustenna, and Invega Hafyera
A drug called Invega comes in several versions: Invega Trinza, Invega Sustenna, and Invega Hafyera. They all contain the active drug paliperidone palmitate. Invega is an extended-release tablet that’s typically taken daily. Invega Sustenna is an IM injection that’s given once per month. Invega Trinza is an IM injection given every 3 months. Invega Hafyera is an IM injection given every 6 months.
This dosage article will focus only on Invega Trinza. Your doctor can tell you more about the other versions.
For information about the dosage of Invega Trinza, including its strengths and how to take the drug, keep reading. For a comprehensive look at Invega Trinza, see this article.
This article describes typical dosages for Invega Trinza provided by the drug’s manufacturer. However, your doctor will prescribe the Invega Trinza dosage that’s right for you.
Invega Trinza is approved to treat schizophrenia in adults who have received Invega Sustenna for at least 4 months. (For details about Invega Trinza’s uses, see this article.)
Invega Trinza form
Invega Trinza comes as a liquid suspension in a prefilled syringe. (A liquid suspension has solid particles in it.) You’ll receive the drug as an intramuscular injection from a healthcare professional. To receive your doses, you’ll go to your doctor’s office or a clinic.
Invega Trinza strengths
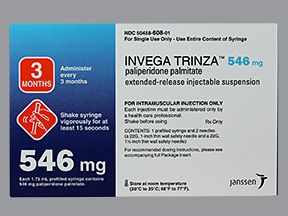
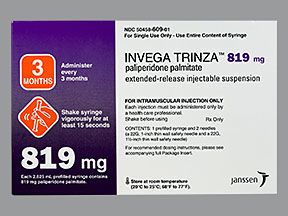
Invega Trinza is available in the following strengths:
- 273 milligrams (mg) of the drug per 0.88 milliliters (mL) of sterile water
- 410 mg/1.32 mL
- 546 mg/1.75 mL
- 819 mg/2.63 mL
Typical dosages
The following information describes dosages that are commonly used or recommended. However, your doctor will administer the dose that’s right for you. They’ll ultimately prescribe the smallest dosage that provides the desired effect.
Invega Trinza dosage for schizophrenia
Invega Trinza has four dosing strengths to treat schizophrenia: 273 mg, 410 mg, 546 mg, and a maximum dose of 819 mg.
Typically, your dose of Invega Trinza will depend on the strength of your last dose of Invega Sustenna. Below is a table to show the standard doses.
| Last dose of Invega Sustenna | Starting dose of Invega Trinza |
| 78 mg | 273 mg |
| 117 mg | 410 mg |
| 156 mg | 546 mg |
| 234 mg | 819 mg |
Children’s dosage
Invega Trinza is not approved for use in children. If your child has schizophrenia, talk with their doctor about treatment options.
Long-term treatment
Invega Trinza is meant to be given as a long-term treatment. If you and your doctor determine that the medication is safe and effective for you, you’ll likely receive it long term.
It’s important not to miss any appointments to receive your Invega Trinza doses. Receiving the medication as scheduled helps keep a steady level of it in your system so it can work effectively. However, there’s a dosing window for Invega Trinza. This window allows you to receive your injection 2 weeks before or 2 weeks after the date of your 3-month appointment.
This dosing window means that Invega Trinza will still work well for you as long as you have your dose within either 2-week period. If you wait beyond the 2-week dosing window, your medication may be less effective than usual.
If you miss an appointment, call your doctor’s office right away to reschedule your missed dose.
If you wait too long, your doctor may need to restart your treatment with Invega Sustenna. If this is needed, your doctor will explain the dosing schedule.
To help make sure that you don’t miss an appointment to receive a dose, try using a medication reminder. This can include setting an alarm or using a timer. You could also download a reminder app on your phone.
The Invega Trinza dosage your doctor prescribes will depend on several factors. These include:
- the severity of the condition you’re using Invega Trinza to treat
- your previous Invega Sustenna dosage
Other medical conditions you have can also affect your Invega Trinza dosage.
Invega Trinza comes as a liquid suspension in a prefilled syringe. (A liquid suspension has solid particles in it.) You’ll receive your dose as an intramuscular injection in your buttocks or upper arm. The drug is given by a healthcare professional at a medical clinic or doctor’s office.
If you have any questions about what to expect with your injections, talk with your doctor.
The dosages in this article are typical dosages provided by the drug’s manufacturer. If your doctor recommends Invega Trinza for you, they’ll prescribe the dosage that’s right for you.
If you have questions about the dosage of Invega Trinza that’s best for you, talk with your doctor.
Besides learning about dosage, you may want other information about Invega Trinza. These additional articles might be helpful:
- More about Invega Trinza. For information about other aspects of Invega Trinza, refer to this article.
- Details about schizophrenia. For details about your condition, see our mental health hub and this list of schizophrenia articles.
Disclaimer: Medical News Today has made every effort to make certain that all information is factually correct, comprehensive, and up to date. However, this article should not be used as a substitute for the knowledge and expertise of a licensed healthcare professional. You should always consult your doctor or another healthcare professional before taking any medication. The drug information contained herein is subject to change and is not intended to cover all possible uses, directions, precautions, warnings, drug interactions, allergic reactions, or adverse effects. The absence of warnings or other information for a given drug does not indicate that the drug or drug combination is safe, effective, or appropriate for all patients or all specific uses.